the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Population dynamics of the Manyara monkey (Cercopithecus mitis manyaraensis) and vervet monkey (Chlorocebus pygerythrus) in Lake Manyara National Park, Tanzania
Christian Kiffner
John Kioko
Thomas M. Butynski
Yvonne A. de Jong
Dietmar Zinner
Estimating population densities and their trends over time is essential for understanding primate ecology and for guiding conservation efforts. From 2011 through to 2019, we counted two guenon species during seasonal road transect surveys in Lake Manyara National Park: the Tanzania-endemic Manyara monkey Cercopithecus mitis manyaraensis (International Union for Conservation of Nature and Natural Resources, IUCN, Red List category of “endangered”) and the vervet monkey Chlorocebus pygerythrus (Red List category of “least concern”). To account for imperfect detectability, we analysed the data in a line distance sampling framework, fitted species-specific detection functions, and subsequently estimated seasonal densities. To test for seasonal differences and yearly trends in the species-specific density estimates, we fitted generalized additive models. Seasonal point density estimates fluctuated considerably during the 9 years (2011–2019) of our study, ranging from 3 to 29 individuals km−2 for Manyara monkeys and from 19 to 83 individuals km−2 for vervet monkeys. Densities of both taxa did not differ seasonally, and we did not detect marked directional population trends. Our study illustrates the utility and limitations of line distance sampling for long-term primate monitoring. Beyond informing primate ecology and management, our results highlight the conservation importance of Lake Manyara National Park for primate conservation.
- Article
(1373 KB) - Full-text XML
- BibTeX
- EndNote
In tropical forests, primates constitute a major component of animal communities and interact with the environment and other species in many ways as folivores, frugivores, omnivores, insectivores, seed dispersers, seed predators, pollinators, and predators or prey (Chapman et al., 1999; Chapman and Dunham, 2018). Primates often contribute substantially to the vertebrate biomass and perform ecological services that are important for the maintenance of tropical forests (Oates et al., 1990; Hall et al., 2003; Fashing et al., 2012). In particular, primates are critical seed dispersers across the forest ecosystems of Africa, Madagascar, and South America (Janson, 1983; Bourlière, 1985; Gautier-Hion et al., 1985; Chapman, 1995; Jordano, 1995; Dew and Wright, 1998; Ganzhorn et al., 1999).
Due to substantial anthropogenic pressures, more than half (60 %) of the world's primate species are threatened with extinction, and an even greater percentage of primate species (75 %) exhibit declining populations (Estrada et al., 2017). Assessing the ecological impact of primates, as well as their conservation status, requires reliable estimates of their population densities or abundances over long time periods (Chapman et al., 2010). Monitoring of primate species is, therefore, particularly important for endemic species that occur in landscapes that are subject to rapid anthropogenic change.
Lake Manyara National Park (hereafter referred to as “LMNP”) is home to five primate species: the Pangani small-eared galago (Otolemur garnettii panganiensis); the Uganda lesser galago (Galago senegalensis sotikae); the olive baboon (Papio anubis); and two species of guenon, the East Africa vervet monkey (Chlorocebus pygerythrus) (hereafter referred to as “vervet monkey”), a widespread species in East Africa, and the gentle monkey (Cercopithecus mitis) (Foley et al., 2014; Kiffner et al., 2015b). Recent molecular findings (Zinner et al., 2022), the examination of many hundreds of wild vervets across East Africa and of museum specimens (including holotypes and paratypes), and a review of the literature indicate that the vervet of this region is not Hilgert's vervet (Chlorocebus pygerythrus hilgerti) as often stated in the latest literature, but its taxonomy is not certain. The International Union for Conservation of Nature and Natural Resources (IUCN) Red List category of threat for C. pygerythrus is “least concern” (Butynski and De Jong, 2022). Recently, the region's gentle monkey (i.e. occurring in LMNP, the forests of the Ngorongoro Conservation Area, and some remnant forests in the Burunge Wildlife Management Area and Karatu District) has been described as a new subspecies, “Manyara monkey” Cercopithecus mitis manyaraensis (Butynski and De Jong, 2020). IUCN lists this subspecies as “endangered” and declining (De Jong and Butynski, 2020). Due to its conservation status, small geographic range (ca. 1480 km2; (Butynski and De Jong, 2020), and suspected population decline, information on the size and dynamics of this population is required to guide conservation efforts.
LMNP (680 km2 with a 460 km2 dry land area), in central northern Tanzania, has experienced manifold human-induced changes in recent decades (Mwalyosi, 1981; Prins and Douglas-Hamilton, 1990; Kiffner et al., 2017). Due to human population growth and changes in livelihood strategies, LMNP is increasingly isolated from adjacent protected areas (Newmark, 1996; Msoffe et al., 2011). Several large mammal species, including black rhinoceros (Diceros bicornis), eland (Taurotragus oryx), hartebeest (Alcelaphus buselaphus), and wild dog (Lycaon pictus), have been extirpated in recent decades (Kiffner et al., 2015b; Steinbeiser et al., 2019). Anthrax (Bacillus anthracis) and rinderpest (genus Morbillivirus) epidemics have caused die-offs among wild ruminants (Prins and Weyerhaeuser, 1987), and yaws (Treponema pallidum pertenue) circulates within the park's primate populations. In olive baboons, this bacterial infection causes ulcerative anogenital skin lesions and affects reproductive behaviour (Knauf et al., 2018; Paciência et al., 2019).
Water flow from catchment areas has markedly declined (Prins and de Jong, 2022), but floods occur more frequently, leading to silting of the lake (Janssens de Bisthoven et al., 2020). The once predominantly open savannah habitats have transitioned into woodlands with a dense understorey (Kiffner et al., 2017). In addition, the boundaries of the park have been extended to the south and southwest. Concomitantly with these often cascading and interrelated changes, population densities of large herbivorous mammals have changed dynamically, with some species declining and others increasing (Kiffner et al., 2017). Whereas bush encroachment may be beneficial for some primate species, it is unclear how the sum of the various perturbations affects the population dynamics of primate species in LMNP.
In contrast to the predominantly arboreal Manyara monkey, the semi-terrestrial vervet monkey might be differentially affected by the change in LMNP habitats. Therefore, we compare the population trajectories of both species and present seasonal density estimates of Manyara monkey and vervet monkey in LMNP over 9 years from November 2011 to November 2019. Based on time series of species-specific density estimates and accounting for potential seasonal effects, we assess density trends. In addition, we provide data on the spatial distribution and group sizes of both species in LMNP.
Prior to fieldwork, we obtained permission (permit nos. 2012-241-NA-2012-57 through 2020-130-NA-2013-191) to conduct this research from the Tanzanian Wildlife Research Institute (TAWIRI) and the Tanzania Commission for Science and Technology (COSTECH). Due to the observational nature of this study, no ethical consent was required.
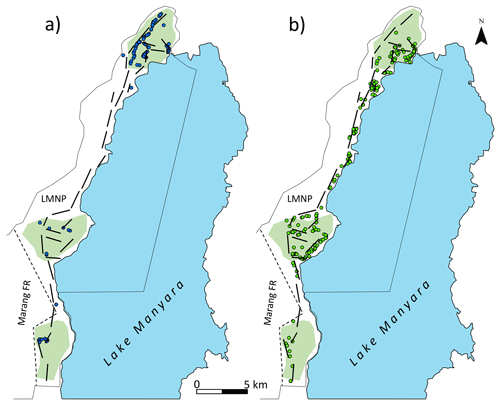
Figure 1Map of Lake Manyara National Park (LMNP): thin black lines denote the border of the national park; dashed lines denote the border between the old park and the park extension (Marang Forest); thick black lines represent transects; light green polygons show the approximate position and size of forests, extracted from Loth (1999) and CCI Land Cover (2016); (a) blue dots represent sightings of Manyara monkeys during 2015–2019 surveys; and (b) green dots represent sightings of vervet monkeys during 2015–2019 surveys. Base map by Esri.
2.1 Lake Manyara National Park
We conducted fieldwork in the lowland area of LMNP (ca. 950–1100 m a.s.l.), a narrow stretch of land (ca. 168 km2) between the western escarpment of the Eastern (Gregory) Rift valley and the shallow and alkaline Lake Manyara (Fig. 1). The climate is semi-arid to semi-humid with a bimodal rainfall pattern. From 2011 through 2018, annual rainfall at LMNP headquarters ranged from 430 to 1060 mm. Most rain occurs during February–May (long rains) and November–December (short rains; Prins and Loth, 1988). Relatively fertile soils, local rainfall, and an extensive surface and groundwater water influx from the Karatu Highlands ensure high primary productivity (Prins, 1988). LMNP is covered by multiple habitat types, including alkaline grasslands near the lake, Vachellia (Acacia) woodlands, and escarpment woodlands (Greenway and Vesey-Fitzgerald, 1969; Loth and Prins, 1986; Prins, 1988; Butynski and De Jong, 2020). In addition, there are groundwater forests and riverine forests where, among other tree species, sycamore figs (Ficus sycomorus) grow. Relatively extensive stands of fever trees (Vachellia xanthophloea) are often situated adjacent to patches of groundwater forest. In response to multiple ecological and anthropogenic changes over the last 40 years, the vegetation density of the bush layer of wooded habitats has substantially increased (Kiffner et al., 2017). However, the park still hosts a relatively diverse large-mammal community (Foley et al., 2014), including predators of primates such as the leopard (Panthera pardus) and lion (Panthera leo) (Palombit, 2013; Isbell et al., 2018; Havmøller et al., 2021). In addition, raptors that prey on guenons, such as the crowned eagle (Stephanoaetus coronatus), martial eagle (Polemaetus bellicosus), and Verreaux's eagle (Aquila verreauxii), are present (Cordeiro, 1992; Isbell and Estam Jaffe, 2013; Lawes et al., 2013; McPherson et al., 2016; Paciência et al., 2017) .
2.2 Line distance surveys
We counted Manyara monkeys and vervet monkeys during 2011–2019 as part of a multi-species line-transect monitoring programme in the lowland area of LMNP (Kiffner et al., 2020). We distributed multiple 2 km long transects along existing roads (Fig. 1). We aimed to repeat all transects each survey, but this was not always possible due to poor road conditions or road closures. The number of transects per survey ranged from 23 to 39. Consecutive transects were separated by 0.5 km to increase independence among transects. The transect length and distance between transects were measured using the vehicle's odometer. We used road transects because off-road restrictions did not allow for a systematically distributed transect layout. Generally, we surveyed these transects three times per year: during the long rains (LR), the dry season (Dry), and the short rains (SR). However, because we started the monitoring at the end of 2011, we only conducted one survey in 2011, and we also skipped the dry season survey in 2019. We conducted LR surveys from the end of February into early April, dry season surveys during July–August, and SR surveys from the end of September into November. The majority of observers were undergraduate students of a study abroad programme focussing on wildlife management. Prior to the survey, all participants were trained in species identification as well as in the theoretical and practical aspects of distance sampling methodology. Moreover, each observer team was accompanied by at least one staff member with extensive experience in species identification and wildlife survey methodology. Due to variation in the cohort sizes of semester groups, the number of observers varied across vehicles and surveys, ranging from 3 to 9 observers per vehicle. To conduct these diurnal surveys (usually carried out between 8:00 and 18:00 EAT), we slowly (5–20 km h−1) drove along transects in open-top Toyota Land Cruiser vehicles and stopped when the driver or observers detected a target species. We then counted individuals using a 50 m threshold for inter-individual distances to define a cluster (Kasozi and Montgomery, 2020). Clusters in distance sampling refer to groupings of organisms that fulfil a certain criterion of spatial organization (here, the 50 m threshold). Clusters can contain one or multiple organisms. Subsequently, we measured the perpendicular distance between the transect and the centre of the cluster with a laser range finder (Bushnell Elite 1500). If monkeys moved before the vehicle was in a position that was perpendicular to the cluster, we measured the perpendicular distance between the transect and the initial location of the cluster (Buckland et al., 2001).
In all surveys, we completed all transects within 1 d using one to five vehicles. If multiple vehicles were used, we assigned non-overlapping sections of LMNP to each vehicle to minimize double-counting due to animal movement. In some seasonal surveys, we repeated transects up to three times within a few days. In these cases, we combined the effort and sightings on the same transects before estimating densities (e.g. if a 2 km transect was driven three times during a few days, the line length was entered as 6 km and all sightings were associated with the unique identification of this transect). This is the procedure recommended for these cases to avoid pseudoreplication (Buckland et al., 2001). From the 2015 SR survey onwards, we collected GPS coordinates of primate sightings and used these geo-referenced sightings to illustrate the distribution of the two target species on a map (Fig. 1).
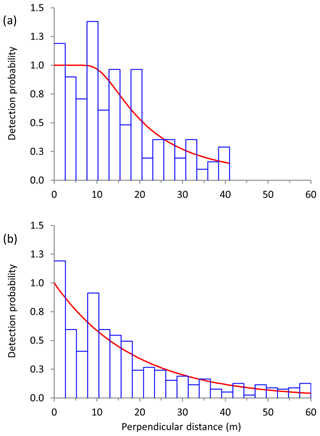
Figure 2Frequency of sightings (blue histogram) at each perpendicular distance bin as well as the fitted detection functions (red line) for (a) Manyara monkeys (conventional distance sampling, hazard rate key function with cosine series expansions) and (b) vervet monkeys (conventional distance sampling, negative exponential key function with cosine series expansions) along road transects in Lake Manyara National Park, Tanzania.
2.3 Data analysis
To estimate the population densities of the two species, we analysed the data in the line distance framework – a method that explicitly accounts for imperfect detectability of the focal species (Buckland et al., 2001; Thomas et al., 2010). For each species, we tested six candidate detection models in the Distance 6.0 program. To base the detection models on a sufficient sample size, we modelled global detection models based on the pooled sightings of each species during 2011–2019 (Manyara monkey: 286 observed clusters; vervet monkey: 525 observed clusters), after discarding 10 % of the farthest sightings from the transect. For each of the six species-specific key functions, we considered cosine expansion series, used estimates of average cluster sizes obtained during each survey, and utilized the post-stratification option to estimate season-specific densities. Among conventional distance sampling (CDS) models, we fitted uniform, half-normal, hazard rate, and negative exponential key functions. Considering that seasonality may have affected detectability (e.g. due to plant phenology, one may suspect detectability to be lower during the rainy seasons compared with the dry season), we additionally ran two multiple-covariate distance sampling (MCDS) models. We parameterized these models with either a half-normal or hazard rate key function. As a model selection statistic, we considered Akaike information criterion (AIC) scores and ranked the six competing models accordingly. As additional criteria, we considered formal model fit statistics and visual fit of the models (Buckland et al., 2001).
To illustrate population densities and their trends over time, we plotted seasonal density estimates (and the associated 95 % CI) using the “ggplot2” package (Wickham, 2016) implemented in R 4.1.1 (R Core Team, 2021). To assess if the densities of the two species differed seasonally and annually, we fitted a general additive model (GAM) to the mean seasonal density estimates using the “mgcv” package (Wood, 2016). In these GAMs, we defined “season” as a categorical variable with three levels and modelled the annual trend-year with a smooth term for the year. The dimension of the basis (k) used for the smooth term “year” was set to the maximum value for the duration of the study (i.e. 9) to allow sufficient “wiggliness” of the trend line. To test for seasonal variation in cluster sizes, we used a negative binomial regression model (Zeileis et al., 2008).
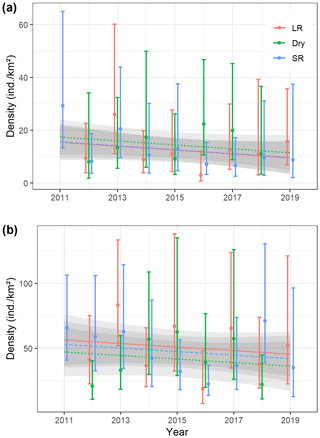
Figure 3Seasonal (LR – long rains, Dry – dry season, and SR – short rains) density estimates (error bars indicate 95 % confidence intervals) of (a) Manyara monkeys and (b) vervet monkeys in Lake Manyara National Park, Tanzania, during 2011–2019 (one survey in 2011, three surveys per year from 2012 to 2018, and two surveys in 2019), and seasonal trend lines based on a general additive model.
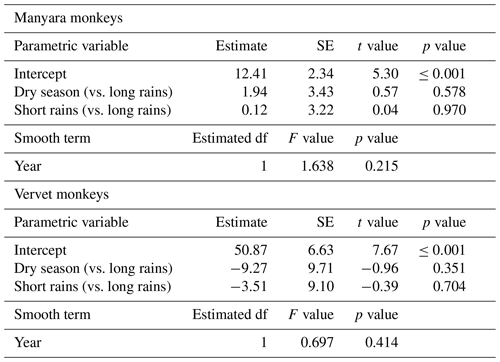
For Manyara monkeys, model selection suggested that a model with a hazard rate key function (Table 1) best explains detection as a function of perpendicular distance from the transect line. While the Kolmogorov–Smirnov test statistic indicates issues with model fit (this holds for all models; Table 1), the visual fit of the model appears good (Fig. 2a). The three most supported detection models – hazard rate, uniform, and negative exponential – yielded relatively similar average densities across the study period: 13.0 [95 % CI: 10.2, 16.7], 14.4 [11.3, 18.5], and 15.0 [11.0, 20.4] individuals km−2 respectively.
Seasonal density estimates are associated with relatively wide margins of error (Fig. 3a); coefficient estimates of the GAM suggest neither significant seasonal density differences nor a marked annual trend in Manyara monkey densities (Table 2). Manyara monkeys occurred unevenly across the study area (Fig. 1a). We detected them mostly in the northern section (dominated by groundwater forests), in the central section near the Endabash River (characterized by riverine forests), and in the southern section (characterized by riverine forests).
Table 1Key parameters associated with six detection models for Manyara monkeys and vervet monkeys in Lake Manyara National Park, Tanzania. “Pa” is the estimated detection probability; “ESW” is the estimated strip width in metres; “KS p value” is the probability of a Kolmogorov–Smirnov goodness of fit test. The numbers in brackets denote the 95 % confidence intervals.
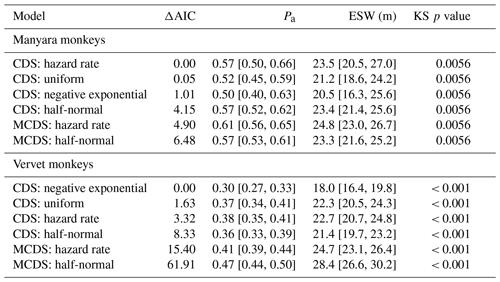
For vervet monkeys, model selection suggests a model with a negative exponential key function to best describe the detection process (Table 1). Similar to the Manyara monkey model, the Kolmogorov–Smirnov test indicates a poor model fit (Table 1), but the visual fit of the model appears relatively good (Fig. 2b) except for some deviations between fitted and observed values in the first distance bins (0–10 m from the transect). The best supported detection model yielded greater average vervet monkey densities across the study period (46.8 [95 CI: 39.6, 55.5] individuals km−2) than the second ranked model (uniform: 37.9 [32.1, 44.7] individuals km−2) and third ranked model (hazard rate: 37.3 [31.5, 44.1] individuals km−2).
The GAM results suggest that densities of vervet monkeys neither differed systematically by season nor showed a significant annual trend over the survey period (Table 2; Fig. 3b). In contrast to Manyara monkeys, vervet monkeys were widely distributed across LMNP (Fig. 1b).
Table 2Parameter estimates of generalized additive models to describe annual and seasonal trends of Manyara monkey and vervet monkey densities in Lake Manyara National Park, Tanzania. For the smooth term, we state the estimated degree of freedom (df).
Cluster sizes of vervet monkeys (median cluster size of 5 and range of 1–38) were greater than cluster sizes of Manyara monkeys (median of 3 and range of 1–27; Fig. 4). Based on a negative binomial regression model, average cluster sizes of Manyara monkeys (Fig. 4a) were significantly (p=0.01) greater during the short rains than during the dry season. Cluster size did not differ significantly (p=0.78) between the dry season and long rains. In contrast, cluster sizes of vervet monkeys were significantly greater during the long rains (p=0.02) and did not differ significantly (p=0.09) between the short rains and the dry season.
Our analyses of line-transect surveys from 2011 to 2019 suggest that population densities both of Manyara monkey and vervet monkey remained fairly stable in the surveyed section of LMNP. While vervet monkeys were widely distributed across the surveyed area and occurred at greater densities than Manyara monkeys, Manyara monkeys were, as expected, more restricted to the forested areas of LMNP. In the following, we discuss the observed population dynamics and highlight the conservation implications of these results with a particular focus on the endangered Manyara monkey.
4.1 Population dynamics
As expected for primate species that occupy relatively stable home ranges throughout the year (Struhsaker, 1967; Cords, 1986; Butynski, 1990; Isbell et al., 1990, 2018; Fashing and Cords, 2000), we did not find marked density differences across the three seasons. In terms of cluster sizes, we found significant differences in both species (Fig. 4), yet the magnitude of these differences was relatively small. Importantly, our monitoring data and subsequent trend analyses suggest that population densities of both species have remained fairly stable over the surveyed period (Fig. 3; see Table 2 for model coefficients). While this sounds positive at a time when primate populations are struggling around the globe (Estrada et al., 2017), we caution against overly optimistic interpretations of our time series. Firstly, seasonal density estimates for both species are associated with a substantial amount of uncertainty (indicated by wide 95 % confidence intervals), making it difficult to detect significant annual trends. Secondly, the overall trend lines indicate a slight downwards direction in both species (Fig. 3). In contrast to several larger mammal species in LMNP, such as savannah elephants (Loxodonta africana), African buffalo (Syncerus caffer), plains zebra (Equus quagga), impala (Aepyceros melampus), or olive baboons (Kiffner et al., 2017), we lack baseline density data for both guenon species before major perturbations occurred in LMNP (i.e. prior to the 1980s). Therefore, elucidating whether the populations of Manyara monkey and vervet monkey have declined, increased, or remained largely unaffected by the substantial changes that occurred in LMNP over recent decades (Prins and de Jong, 2022) cannot be established with certainty.
4.2 Primate densities in context
Population densities of gentle monkeys and vervet monkeys have been estimated at various sites in eastern Africa (Struhsaker, 1967; Butynski, 1990; Isbell et al., 1990, 1999; Thomas, 1991; Plumptre and Reynolds, 1994; Lee and Hauser, 1998; Fashing and Cords, 2000; Hall et al., 2003; Uehara, 2003; Fashing et al., 2012; McLester et al., 2019). As field survey and analytical methods varied among sites, it is, however, difficult to compare estimates among studies. Furthermore, comparative studies on olive baboon survey methods in LMNP provide evidence that distance sampling along roads yields biased absolute population density estimates (Kiffner et al., 2022). Manyara monkey densities (average of 13.0 individuals km−2; 95 % CI: 10.2–16.7) for LMNP are, at least, well within the range of densities for gentle monkeys at other sites in central Africa and eastern Africa (Butynski, 1990; Thomas, 1991; Plumptre and Reynolds, 1994; Fashing and Cords, 2000; Hall et al., 2003; Uehara, 2003; Fashing et al., 2012; McLester et al., 2019). Similarly, densities of vervet monkeys estimated for LMNP (46.8 individuals km−2; 95 % CI: 39.6–55.5) are also comparable to densities at other sites in East Africa (Struhsaker, 1967; Isbell et al., 1990, 1999; Lee and Hauser, 1998).
In contrast to Manyara monkeys, vervet monkeys were widely distributed across LMNP and occurred in all habitat types (Fig. 1b). Although both species are considered diet generalists (Butynski, 1990; Isbell and Estam Jaffe, 2013; Lawes et al., 2013), the arboreal Manyara monkey largely depends on closed forest, whereas the semi-terrestrial vervet monkey largely depends on woodland and forest edge (Fig. 1). The wide distribution of vervet monkeys in LMNP was further confirmed by systematic camera trapping: an occupancy model suggests that vervet monkeys occupied approximately 74 % of the lowland area (Steinbeiser et al., 2019). The greater dependence on evergreen forests explains the heterogeneous distribution of Manyara monkeys within LMNP (Fig. 1a). In other areas, substantial heterogeneity of gentle monkey densities at relatively small spatial scales has been observed and attributed to perturbations such as disease outbreaks (Butynski, 1990). Although illegal hunting for bushmeat affects several larger mammal species in LMNP (Kiffner et al., 2017), primates are generally not hunted in this region (Kiffner et al., 2015a). Monkeys are unlikely the target species of poachers here, but they can be a by-catch of snares, as reported for southern patas monkeys (Erythrocebus baumstarki) in the western Serengeti (De Jong and Butynski, 2021). While we cannot rule out possible impacts of diseases on gentle monkey populations (e.g. yaws circulates in Manyara monkeys; Chuma et al., 2018), we strongly suspect that the limited availability of evergreen forest habitat predominantly determines population size. In LMNP, a system characterized by a patchy distribution of diverse habitat types (Loth and Prins, 1986), systematic camera trapping (16 % occupancy; Steinbeiser et al., 2019) and systematic line-transect surveys (this study) all indicate a spatially restricted distribution of Manyara monkeys that is tightly linked to groundwater forests and riverine forests (Fig. 1a). As the gentle monkey is a food generalist, with an emphasis on fruit and invertebrates (Cords, 1986; Beeson, 1989; Butynski, 1990; Kaplin et al., 1998; Takahashi et al., 2019), we speculate that the availability of food, especially fruits, coincides with these habitat types and explains the restricted distribution of Manyara monkeys in LMNP. This, in turn, explains the low population densities compared with other study areas (e.g. Kakamega Forest in Kenya and Kibale Forest in Uganda) which have greater proportions of evergreen closed forest and relatively little savannah habitat. In addition, competition with the abundant and widely distributed vervet monkey (Fig. 1b) and olive baboon (Kiffner et al., 2022) might influence their distribution. Specific patterns of primate co-occurrences in LMNP are known (i.e. significant associations between Manyara monkeys and vervet monkeys occur, whereas no significant associations between Manyara monkeys and baboons have been found; Kiffner et al., 2014). However, little is known about fitness-relevant repercussions (e.g. competition vs. facilitation) of interspecific interactions among primate species in LMNP and whether these interactions influence primate distributions and abundance. Although we cannot exclude a possible influence of methodology on density estimates, interspecific variation seems strongly influenced by ecological variables such as habitat quality and possibly disease, predation, and other factors (Whitten, 1982; Butynski, 1990).
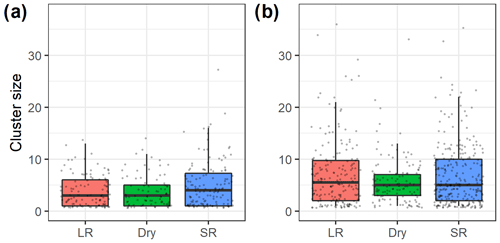
Figure 4Box plots (bold midline indicates the median, and the upper and lower limits of the box represent the respective third and first quartiles) of (a) Manyara monkey and (b) vervet monkey cluster sizes during seasonal (LR – long rains, Dry – dry season, and SR – short rains) line distance surveys in Lake Manyara National Park, Tanzania. The grey dots represent individual data points.
4.3 Conservation implications
Before providing further interpretations of our data for conservation assessments, a word of caution is appropriate. As our density estimates are based on road surveys (which may not represent the entire study area; Kiffner et al., 2022) and as both monkey species showed distinct distribution patterns (which are not accounted for when extrapolating densities from the area covered by the transect to the study area), the actual population density of the surveyed species may differ from our estimates. Potential design-based bias probably does not affect temporal trends, but the non-random distribution of transects can substantially affect absolute density estimates (Beaver et al., 2014; Kiffner et al., 2017). To provide more accurate estimates of the abundance of Manyara monkeys, we recommend dedicated surveys in their preferred habitats (e.g. groundwater forest and riverine forest; Fig. 1a). Such targeted surveys are necessary to provide a clearer basis for assessing the conservation status of the Manyara monkey.
As mentioned above, the Manyara monkey has recently been described (based on their unique external phenotype and isolated geographic range) as a subspecies within the gentle monkey complex (Butynski and De Jong, 2020). This subspecies occurs within a relatively restricted area of northern Tanzania (known distribution range of 1480 km2 and a probable range of 5865 km2). The range includes the forests of two protected areas (i.e. Ngorongoro Conservation Area and LMNP) that are likely the main strongholds of this subspecies (Butynski and De Jong, 2020). The Manyara monkey is listed as “endangered” on “The IUCN Red List of Threatened Species” due to continuing habitat loss, degradation, and fragmentation in their small geographic range (De Jong and Butynski, 2020). In light of the restricted distribution of this subspecies within the study area in LMNP and the associated spatial heterogeneity in density, we strongly caution against extrapolating our density estimates to the suggested distribution range. Overall, we echo the recommendations of Butynski and De Jong (2020) with respect to continued effective protected area management. In particular, we assume that effectively protecting riverine and groundwater forests is crucial for the long-term conservation of the Manyara monkey. This subspecies also occupies areas outside protected areas, such as the remnant communal riverine forests on the Mbulu Plateau in the Endabash area of Karatu District, and communal land along the Rift Valley escarpment to the north of LMNP. This monkey also occasionally moves through the agricultural matrix between LMNP and the Ngorongoro Conservation Area (Christian Kiffner, personal observation, 1 March 2020). Thus, protecting existing forested wildlife corridors, such as the Upper Kitete Corridor (which links the Ngorongoro Conservation Area to LMNP), as well as riverine forest and ground water forest on communal land, will help ensure the persistence of this subspecies across its current range (Butynski and De Jong, 2020).
Data on population density estimates and observed cluster sizes are publicly available at Göttingen Research Online: https://doi.org/10.25625/95WCN8 (Kiffner, 2022).
CK and JK designed the study and collected the data. CK and DZ analysed the data and prepared the manuscript with contributions from all co-authors.
At least one of the (co-)authors is a member of the editorial board of Primate Biology. The peer-review process was guided by an independent editor, and the authors also have no other competing interests to declare.
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors wish to thank the Tanzanian Wildlife Research Institute (TAWIRI) and the Tanzania Commission for Science and Technology (COSTECH) for granting permission to do this research. We are also grateful to Tanzania National Parks (TANAPA) at Lake Manyara for access to the study site. The authors sincerely thank all students and staff of the School for Field Studies for participating in the surveys. We also wish to acknowledge Jean-Baptiste Leca and one anonymous reviewer for their constructive comments.
The publication of this article was funded by the Open Access Fund of the Leibniz Association.
This paper was edited by Ute Radespiel and reviewed by Jean-Baptiste Leca and one anonymous referee.
Beaver, J. T., Harper, C. A., Kissel Jr., R. E., Muller, L. I., Basinger, P. S., Goode, M. J., Van Manen, F. T. Winton, W., and Kennedy, M. L.: Aerial vertical-looking infrared imagery to evaluate bias of distance sampling techniques for white-tailed deer, Wildl. Soc. Bull., 38, 419–427, https://doi.org/10.1002/wsb.410, 2014.
Beeson, M.: Seasonal dietary stress in a forest monkey (Cercopithecus mitis), Oecologia, 78, 565–570, https://doi.org/10.1007/BF00378749, 1989.
Bourlière, F.: Primate communities: Their structure and role in tropical ecosystems, Int. J. Primatol., 6, 1–26, https://doi.org/10.1007/BF02693694, 1985.
Buckland, S. T., Anderson, D. R., Burnham, K. P., Laake, J. L., and Borchers, D. L.: Introduction to Distance Sampling. Estimating Abundance of Biological Populations, Oxford University Press, Oxford, UK, ISBN 019850649X, 2001.
Butynski, T. M.: Comparative ecology of blue monkeys (Cercopithecus mitis) in high- and low-density subpopulations, Ecol. Monogr., 60, 1–26, https://doi.org/10.2307/1943024, 1990.
Butynski, T. M. and De Jong, Y. A.: Taxonomy and biogeography of the gentle monkey Cercopithecus mitis Wolf, 1822 (Primates: Cercopithecidae) in Kenya and Tanzania, and designation of a new subspecies endemic to Tanzania, Primate Conserv., 34, 1–48, https://bit.ly/3yZ22IT, (last access: 11 April 2022), 2020.
Butynski, T. M. and De Jong, Y. A.: Chlorocebus pygerythrus, The IUCN Red List Threatened Species, 2022, e.T136271A205998680, https://doi.org/10.2305/IUCN.UK.2022-1.RLTS.T136271A205998680.en, 2022.
CCI Land Cover: S2 prototype Land Cover 20m map of Africa 2016, http://2016africalandcover20m.esrin.esa.int/ (last access: 10 January 2022), 2016.
Chapman, C. A.: Primate seed dispersal: coevolution and conservation implications, Evol. Anthropol., 4, 74–82, https://doi.org/10.1002/evan.1360040303, 1995.
Chapman, C. A. and Dunham, A. E.: Primate seed dispersal and forest restoration: an African perspective for a brighter future, Int. J. Primatol., 39, 427–442, https://doi.org/10.1007/s10764-018-0049-3, 2018.
Chapman, C. A., Gautier-Hion, A., Oates, J. F., and Onderdonk, D. A.: African primate communities: determinants of structure and threats to survival, in: Primate Communities, edited by: Fleagle, J. G., Janson, C., and Reed, K., Cambridge University Press, Cambridge, UK, 1–37, https://doi.org/10.1017/CBO9780511542381.002, 1999.
Chapman, C. A., Struhsaker, T. T., Skorupa, J. P., Snaith, T. V., and Rothman, J. M.: Understanding long-term primate community dynamics: implications of forest change, Ecol. Appl., 20, 179–191, https://doi.org/10.1890/09-0128.1, 2010.
Chuma, I. S., Batamuzi, E. K., Collins, D. A., Fyumagwa, R. D., Hallmaier-Wacker, L. K., Kazwala, R. R., Keyyu, J. D., Lejora, I. A., Lipende, I. F., Lüert, S., Paciência, F. M. D., Piel, A., Stewart, F. A., Zinner, D., Roos, C., and Knauf, S.: Widespread Treponema pallidum infection in nonhuman primates, Tanzania, Emerg. Infect. Dis., 24, 1002–1009, https://doi.org/10.3201/eid2406.180037, 2018.
Cords, M.: Interspecific and intraspecific variation in diet of two forest guenons, Cercopithecus ascanius and C. mitis, J. Anim. Ecol., 55, 811–827, https://doi.org/10.2307/4418, 1986.
Cordeiro, N. J.: Behaviour of blue monkeys (Cercopithecus mitis) in the presence of crowned eagles (Stephanoaetus coronatus), Folia Primatol., 59, 203–206, https://doi.org/10.1159/000156660, 1992.
De Jong, Y. A. and Butynski, T. M.: Cercopithecus mitis ssp. manyaraensis. The IUCN Red List of Threatened Species, e.T95596261A95596266, https://doi.org/10.2305/IUCN.UK.2020-2.RLTS.T95596261A95596266.en, 2020.
De Jong, Y. A. and Butynski, T. M.: Is the southern patas monkey Erythrocebus baumstarki Africa's next primate extinction? Reassessing taxonomy, distribution, abundance, and conservation, Am. J. Primatol., 83, e23316, https://doi.org/10.1002/ajp.23316, 2021.
Dew, J. L. and Wright, P.: Frugivory and seed dispersal by four species of primates in Madagascar's eastern rain forest, Biotropica, 30, 425–437, https://doi.org/10.1111/j.1744-7429.1998.tb00076.x, 1998.
Estrada, A., Garber, P. A., Rylands, A. B., Roos, C., Fernandez-Duque, E., Di fiore, A., Nekaris, K. A.-I., Nijman, V., Heymann, E. W., Lambert, J. E., Rovero, F., Barelli, C., Setechell, J. M., Gillespie, T. R., Mittermeier, R. A., Arregoita, L. V., de Guinea, M., Gouveia, S., Dobrovolski, R., Shanee, S., Shanee, N., Boyle, S. A., Fuentes, A., MacKinnon, K. C., Amato, K. R., Meyer, A. L. S., Wich, S., Sussman, R. W., Pan, R., Kone, I., and Li, B.: Impending extinction crisis of the world's primates: Why primates matter, Sci. Adv., 3, e1600946, https://doi.org/10.1126/sciadv.1600946, 2017.
Fashing, P. J. and Cords, M.: Diurnal primate densities and biomass in the Kakamega Forest: an evaluation of census methods and a comparison with other forests, Am. J. Primatol., 50, 139–152, https://doi.org/10.1002/(SICI)1098-2345(200002)50:2<139::AID-AJP4>3.0.CO;2-N, 2000.
Fashing, P. J., Nguyen, N., Luteshi, P., Opondo, W., Cash, J. F., and Cords, M.: Evaluating the suitability of planted forests for African forest monkeys: a case study from Kakamega forest, Kenya, Am. J. Primatol., 74, 77–90, https://doi.org/10.1002/ajp.21012, 2012.
Foley, C., Foley, L., Lobora, A., De Luca, D., Msuha, M., Davenport, T. R. B., and Durant, S.: A field guide to larger mammals of Tanzania, Princeton University, Press, Princeton, USA, https://doi.org/10.1515/9781400852802, 2014.
Ganzhorn, J. U., Fietz, J., Rakotovao, E., Schwab, D., and Zinner, D.: Lemurs and the regeneration of dry deciduous forest in Madagascar, Conserv. Biol., 13, 794–804, https://doi.org/10.1046/j.1523-1739.1999.98245.x, 1999.
Gautier-Hion, A., Duplantier, J.-M., Quris, R., Feer, F., Sourd, C., Decoux, J.-P., Dubost, G., Emmons, L., Erard, C., Hecketsweiler, P., Moungazi, A., Roussilhon, C., and Thiollay, J.-M.: Fruit characters as a basis of fruit choice and seed dispersal in a tropical forest vertebrate community, Oecologia, 65, 324–337, https://doi.org/10.1007/BF00378906, 1985.
Greenway, P. J. and Vesey-Fitzgerald, D. F.: The vegetation of Lake Manyara National Park., J. Ecol., 57, 127–149, https://doi.org/10.2307/2258212, 1969.
Hall, J. S., White, L., Williamson, L., Inogwabini, B.-I., and Ilambu, O.: Distribution, abundance and biomass estimates for primates within the Kahuzi-Biega lowland and adjacent forest in eastern DRC, Afr. Primates, 6, 35–42, 2003.
Havmøller, R. W., Jacobsen, N. S., Havmøller, L. W., Rovero, F., Scharff, N., and Bohmann, K.: DNA metabarcoding reveals that African leopard diet varies between habitats, Afr. J. Ecol., 59, 37–50, https://doi.org/10.1111/aje.12817, 2021.
Isbell, L. and Estam Jaffe, K.: Chlorocebus pygerythrus Vervet Monkey, in: Mammals of Africa Vol II Primates, edited by: Butynski, T. M., Kingdon, J., and Kalina, J., Bloomsburry, London, UK, 277–283, ISBN 9781408122525, 2013.
Isbell, L. A., Cheney, D. L., and Seyfarth, R. M.: Costs and benefits of home range shifts among vervet monkeys (Cercopithecus aethiops) in Amboseli National Park, Kenya, Behav. Ecol. Sociobiol., 27, 351–358, https://doi.org/10.1007/BF00164006, 1990.
Isbell, L. A., Pruetz, J. D., Musyoka Nzuma, B., and Young, T. P.: Comparing measures of travel distances in primates: Methodological considerations and socioecological implications, Am. J. Primatol., 48, 87–98, https://doi.org/10.1002/(SICI)1098-2345(1999)48:2<87::AID-AJP1>3.0.CO;2-G, 1999.
Isbell, L. A., Bidner, L. R., Van Cleave, E. K., Matsumoto-Oda, A., and Crofoot, M. C.: GPS-identified vulnerabilities of savannah-woodland primates to leopard predation and their implications for early hominins, J. Hum. Evol., 118, 1–13, https://doi.org/10.1016/j.jhevol.2018.02.003, 2018.
Janson, C. H.: Adaptation of fruit morphology to dispersal agents in a Neotropical forest, Science, 219, 187–189, https://doi.org/10.1126/science.219.4581.187, 1983.
Janssens de Bisthoven, L. J., Vanhove, M. P. M., Rochette, A., Hug, J., Henok, S., Nhiwatiwa, T., Casier, B., Kiwango, Y. A., Kaitila, R., Komakech, H., and Brendonck, L.: Social-ecological assessment of Lake Manyara basin, Tanzania: A mixed method approach, J. Environ. Manage., 267, 110594, https://doi.org/10.1016/j.jenvman.2020.110594, 2020.
Jordano, P.: Angiosperm fleshy fruits and seed dispersers: a comparative analysis of adaptation and constraints in plant-animal interactions, Am. Nat., 145, 163–191, https://doi.org/10.1086/285735, 1995.
Kaplin, B. A., Munyaligoga, V., and Moermond, T. C.: The influence of temporal changes in fruit availability on diet composition and seed handling in blue monkeys (Cercopithecus mitis doggetti), Biotropica, 30, 56–71, https://doi.org/10.1111/j.1744-7429.1998.tb00369.x, 1998.
Kasozi, H. and Montgomery, R. A.: Variability in the estimation of ungulate group sizes complicates ecological inference, Ecol. Evol., 10, 6881–6889, https://doi.org/10.1002/ece3.6463, 2020.
Kiffner, C.: Replication data for: Population dynamics of Manyara monkey Cercopithecus mitis manyaraensis and vervet monkey Chlorocebus pygerythrus in Lake Manyara National Park, Tanzania, GRO.data [data set], V1, https://doi.org/10.25625/95WCN8, 2022.
Kiffner, C., Kioko, J., Leweri, C., and Krause, S.: Seasonal patterns of mixed species groups in large East African mammals, PLoS ONE, 9, e113446, https://doi.org/10.1371/journal.pone.0113446, 2014.
Kiffner, C., Peters, L., Strohming, A., and Kioko, J.: Bushmeat consumption in the Tarangire-Manyara ecosystem, Tanzania, Trop. Conserv. Sci., 8, 352–366, https://doi.org/10.1177%2F194008291500800204, 2015a.
Kiffner, C., Wenner, C., LaViolet, A., Yeh, K., and Kioko, J.: From savannah to farmland: effects of land-use on mammal communities in the Tarangire-Manyara ecosystem, Tanzania, Afr. J. Ecol., 53, 156–166, https://doi.org/10.1111/aje.12160, 2015b.
Kiffner, C., Rheault, H., Miller, E., Scheetz, T., Enriquez, V., Swafford, R., Kioko, J., and Prins, H. H. T.: Long-term population dynamics in a multi-species assemblage of large herbivores in East Africa, Ecosphere, 8, e02027, https://doi.org/10.1002/ecs2.2027, 2017.
Kiffner, C., Binzen, G., Cunningham, L., Jones, M., Spruiell, F., and Kioko, J.: Wildlife population trends as indicators of protected area effectiveness in northern Tanzania, Ecol. Indic., 110, 105903, https://doi.org/10.1016/j.ecolind.2019.105903, 2020.
Kiffner, C., Paciência, F. M. D., Henrich, G., Kaitilia, R., Chuma, I. S., Mbaryo, P., Knauf, S., Kioko, J., and Zinner, D.: Road-based line distance surveys overestimate densities of olive baboons, PLoS One, 17, e0263314, https://doi.org/10.1371/journal.pone.0263314, 2022.
Knauf, S., Gogarten, J. F., Schuenemann, V. J., De Nys, H. M., Düx, A., Strouhal, M., Mikalová, L., Bos, K. I., Armstrong, R., Batamuzi, E. K., Chuma, I. S., Davoust, B., Diatta, G., Fyumagwa, R. D., Kazwala, R. R., Keyyu, J. D., Lejora, I. A. V, Levasseur, A., Liu, H., Mayhew, M. A., Mediannikov, O., Raoult, D., Wittig, R. M., Roos, C., Leendertz, F. H., Šmajs, D., Nieselt, K., Krause, J., and Calvignac-Spencer, S.: Nonhuman primates across sub-Saharan Africa are infected with the yaws bacterium Treponema pallidum subsp. pertenue, Emerg. Microbes Infect., 7, 1–4, https://doi.org/10.1038/s41426-018-0156-4, 2018.
Lawes, M. J., Cords, M., and Lehn, C.: Cercopithecus mitis Gentle Monkey (Diademed Monkey, Blue Monkey, Sykes's Monkey), in: Mammals of Africa Vol II Primates, edited by: Butynski, T. M., Kingdon, J., and Kalina, J., Bloomsburry, London, UK, 354–362, ISBN 9781408122525, 2013.
Lee, P. C. and Hauser, M. D.: Long-term consequences of changes in territory quality on feeding and reproductive strategies of vervet monkeys, J. Anim. Ecol., 67, 347–358, https://doi.org/10.1046/j.1365-2656.1998.00200.x, 1998.
Loth, P. E.: The vegetation of Manyara: scale-dependent states and transitions in the African Rift Valley, PhD thesis, Wageningen Universiteit, Wageningen, NL, 1999.
Loth, P. E. and Prins, H. H. T.: Spatial patterns of the landscape and vegetation of Lake Manyara National Park, ITC J., 2, 115–130, 1986.
McLester, E., Pintea, L., Stewart, F. A., and Piel, A. K.: Cercopithecine and colobine abundance across protected and unprotected land in the Greater Mahale Ecosystem, Western Tanzania, Int. J. Primatol., 40, 687–705, https://doi.org/10.1007/s10764-019-00118-6, 2019.
McPherson, S. C., Brown, M., and Downs, C. T.: Diet of the crowned eagle (Stephanoaetus coronatus) in an urban landscape: potential for human-wildlife conflict?, Urban Ecosyst., 19, 383–396, https://doi.org/10.1007/s11252-015-0500-6, 2016.
Msoffe, F. U., Kifugo, S. C., Said, M. Y., Neselle, M. O., Van Gardingen, P., Reid, R. S., Ogutu, J. O., Herero, M., and de Leeuw, J.: Drivers and impacts of land-use change in the Maasai Steppe of northern Tanzania: an ecological, social and political analysis, J. Land Use Sci., 6, 261–281, https://doi.org/10.1080/1747423X.2010.511682, 2011.
Mwalyosi, R. B.: Ecological changes in Lake Manyara National Park, Afr. J. Ecol., 19, 201–204, https://doi.org/10.1111/j.1365-2028.1981.tb00664.x, 1981.
Newmark, W. D.: Insularization of Tanzanian parks and the local extinction of large mammals, Conserv. Biol., 10, 1549–1556, https://doi.org/10.1046/j.1523-1739.1996.10061549.x, 1996.
Oates, J. F., Whitesides, G. H., Davies, A. G., Waterman, P. G., Green, S. M., Dasilva, G. L., and Mole, S.: Determinants of variation in tropical forest primate biomass: new evidence from West Africa, Ecology, 71, 328–343, https://doi.org/10.2307/1940272, 1990.
Paciência, F. M. D., Baluya, D., Mbaryo, P., Knauf, S., and Zinner, D.: Olive baboons' (Papio anubis) response towards crowned eagles (Stephanoaetus coronatus) at Lake Manyara National Park, Primate Biol., 4, 101–106, https://doi.org/10.5194/pb-4-101-2017, 2017.
Paciência, F. M. D., Rushmore, J., Chuma, I. S., Lipende, I. F., Caillaud, D., Knauf, S., and Zinner, D.: Mating avoidance in female olive baboons (Papio anubis) infected by Treponema pallidum, Sci. Adv., 5, eaaw9724, https://doi.org/10.1126/sciadv.aaw9724, 2019.
Palombit, R. A.: Papio anubis Olive Baboon, in: Mammals of Africa Vol II Primates, edited by: Butynski, T. M., Kingdon, J., and Kalina, J., Bloomsburry, London, UK, 233–239, ISBN 9781408122525, 2013.
Plumptre, A. J. and Reynolds, V.: The effect of selective logging on the primate populations in the Budongo Forest Reserve, Uganda, J. Appl. Ecol., 31, 631–641, https://doi.org/10.2307/2404154, 1994.
Prins, H. H. T.: Plant phenology patterns in Lake Manyara National Park, J. Biogeogr., 15, 465–480, https://doi.org/10.2307/2845276, 1988.
Prins, H. H. T. and de Jong, J. F.: The ecohistory of Tanzania's Northern Rift Valley – can one establish an objective baseline as an endpoint for ecosystem restoration?, in: Tarangire: Human-Wildlife Coexistence in a Fragmented Ecosystem, edited by: Kiffner, C., Bond, M. L., and Lee, D. E., Springer, Cham, Switzerland, 129–161, https://doi.org/10.1007/978-3-030-93604-4_7, 2022.
Prins, H. H. T. and Douglas-Hamilton, I.: Stability in a multi-species assemblage of large herbivores in East Africa, Oecologia, 83, 392–400, https://doi.org/10.1007/BF00317566, 1990.
Prins, H. H. T. and Loth, P. E.: Rainfall patterns as background to plant phenology in northern Tanzania, J. Biogeogr., 15, 451–463, https://doi.org/10.2307/2845275, 1988.
Prins, H. H. T. and Weyerhaeuser, F. J.: Epidemics in populations of wild ruminants: anthrax and impala, rinderpest and buffalo in Lake Manyara National Park, Tanzania, Oikos, 49, 28–38, https://doi.org/10.2307/3565551,1987.
R Core Team: R: A language and environment for statistical computing, http://www.r-project.org/ (last access: 11 July 2022), 2021.
Steinbeiser, C. M., Kioko, J., Maresi, A., Kaitilia, R., and Kiffner, C.: Relative abundance and activity patterns explain method-related differences in mammalian species richness estimates, J. Mammal., 100, 192–201, https://doi.org/10.1093/jmammal/gyy175, 2019.
Struhsaker, T. T.: Ecology of vervet monkeys in the Masai-Amboseli Game Reserve, Kenya, Ecology, 48, 891–904, https://doi.org/10.2307/1934531, 1967.
Takahashi, M. Q., Rothman, J. M., Raubenheimer, D., and Cords, M.: Dietary generalists and nutritional specialists: Feeding strategies of adult female blue monkeys (Cercopithecus mitis) in the Kakamega Forest, Kenya, Am. J. Primatol., 81, e23016, https://doi.org/10.1002/ajp.23016, 2019.
Thomas, L., Buckland, S. T., Rexstad, E. A., Laake, J. L., Strindberg, S., Hedley, S. L., Bishop, J. R. B. B., Marques, T. A., and Burnham, K. P.: Distance software: Design and analysis of distance sampling surveys for estimating population size, J. Appl. Ecol., 47, 5–14, 10.1111/j.1365-2664.2009.01737.x, 2010.
Thomas, S. C.: Population densities and patterns of habitat use among anthropoid primates of the Ituri Forest, Zaire, Biotropica, 23, 68–83, https://doi.org/10.2307/2388690, 1991.
Uehara, S.: Population densities of diurnal mammals sympatric with the chimpanzees of the Mahale Mountains, Tanzania: comparison between the census data of 1996 and 2000, Afr. Study Monogr., 24, 169–179, https://doi.org/10.14989/68223, 2003.
Whitten, P. L.: Female reproductive strategies among vervet monkeys, PhD thesis, Harvard University, Cambridge, USA, 1982.
Wickham, H.: ggplot2: Elegant Graphics for Data Analysis, Springer, New York, USA, ISBN 978-3-319-24277-4, 2016.
Wood, S. N.: Package “mgcv”, https://cran.r-project.org/web/packages/mgcv/mgcv.pdf (last access: 2 January 2017), 2016.
Zeileis, A., Kleiber, C., and Jackman, S.: Regression models for count data in R, J. Stat. Softw., 27, 1–25, https://doi.org/10.18637/jss.v027.i08, 2008.
Zinner, D., Knauf, S., Chuma, I. S., Butynski, T. M., De Jong, Y. A., Keyyu, J. D., Kaitila, R., and Roos, C.: Mito-phylogenetic relationship of the new subspecies of gentle monkey Cercopithecus mitis manyaraensis, Butynski & De Jong, 2020, Primate Biol., 9, 11–18, https://doi.org/10.5194/pb-9-11-2022, 2022.




