Group size experiences with enhanced pre- and postnatal development studies in the long-tailed macaque (Macaca fascicularis)
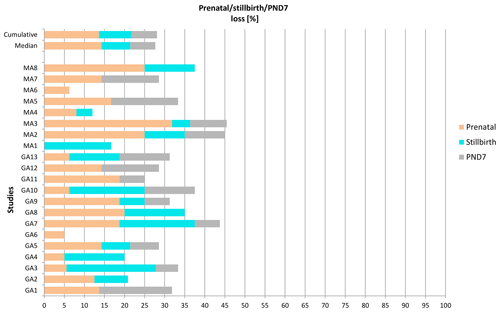
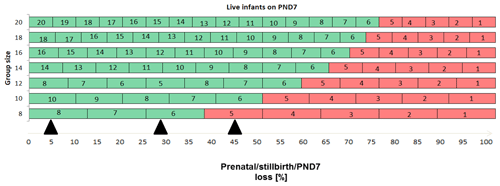
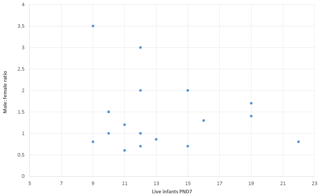
Enhanced pre- and postnatal development (ePPND) studies have become the default developmental toxicity test for biopharmaceuticals if nonhuman primates represent the relevant species. Spontaneous pregnancy losses and infant deaths can be significant in macaques such as long-tailed macaques. The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guideline S6(R1) states that pregnancy outcome can be judged also by the normogram-based variability of reference data according to a publication by Jarvis et al. (2010) defining a study as valid with six to eight live infants in the control group on postnatal day 7 (PND7). Since the release of ICH S6(R1) (2011), ePPND studies for biologics have replaced the former separate embryo-fetal and PPND study types. This work provides a retrospective analysis of pregnancy outcomes from 21 ePPND studies and group sizes of 14–24 animals per group. All studies reached the goal of at least six to eight infants on PND7, with overall losses ranging between 5 % and 45 %. Consistently, a group size of 14–24 maternal animals yielded more than six to eight infants on PND7. Therefore, it is suggested to reduce ePPND study group sizes from 20 to 14, yielding an animal number reduction of approx. 30 %.